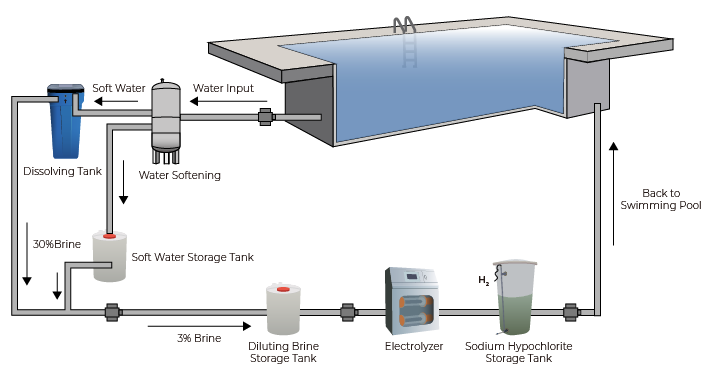
Swimming Pool Disinfection
Electro-chlorination technology has been introduced to this field in the past decade, it has proved to be a longer-term, more cost-effective solution and has better bactericidal effect in comparison with traditional Trichloroisocyanuric acid tablets or powder. Electrolysis systems are a particularly attractive alternatives for safe disinfection as by producing the disinfectant on-site, this systems removes any need to store and handle hazardous chemicals.
Municipal water will first be deionized (DI) to remove any metal cations present (e.g. Mg2+, Ca2+). DI water will then be stored to either dissolve sodium chloride, forming saturated brine, or for brine dilution. The brine solution is diluted to precisely 3% and then transferred to the electrolyzer (sodium hypochloride generator). A detailed electrochemical reactions for this process is laid out below:
Anode: 2Cl– —– Cl2+2e–
Cathode: 2H2O+2e–—–2OH–+H2
Solution Reaction: Cl2+2OH–—–Cl–+ClO–+H2O